Struggling with outsourced clinical trials?
You’re not alone.
Clinical trials often face critical challenges that affect their success and efficiency.
Inefficient or flawed study design, leading to complications in data collection
Inaccurate, incomplete, or inconsistent data
Invalid results due to poor data quality
Insufficient oversight and lack of traceable documentation
Inconsistent data storage leading to potential compliance risks
We support you with our expertise in avoiding these challenges.

Our Services
Efficient study oversight – clear, flexible, reliable.
Whether it’s strategic guidance, document review or real-time oversight – we bring structure to your processes and confidence to your decisions.

Clinical trial oversight
Our Octovis dashboard gives you clarity before things get critical: Early warning indicators, clean visualizations, and audit-ready summaries – true oversight made simple, for traceable results and high-quality documentation.
Review & Quality Assurance
We review what matters: SOPs, Data Management Plan, TLFs, and Clinical Study Reports – for highest data quality.
Consulting & Strategic Support
From CRO selection and protocol and e-CRF design to CTMS implementation – we offer expert input, tailored to your needs.
Collaboration that fits your workflow
Whether you need targeted support, full-scale involvement, or embedded project work – we adapt to your setup.
Efficient study oversight – clear, flexible, reliable.
Whether it’s strategic guidance, document review or real-time oversight – we bring structure to your processes and confidence to your decisions.
Consulting & Strategic Support
From CRO selection and protocol and e-CRF design to CTMS implementation – we offer expert input, tailored to your needs.

Clinical trial oversight
Our Octovis dashboard gives you clarity before things get critical: Early warning indicators, clean visualizations, and audit-ready summaries – true oversight made simple, for traceable results and high-quality documentation.
Review & Quality Assurance
We review what matters: SOPs, Data Management Plan, TLFs, and Clinical Study Reports – for highest data quality.
Collaboration that fits your workflow
Whether you need targeted support, full-scale involvement, or embedded project work – we adapt to your setup.
Curious to learn more?
Let’s talk – and find the best path forward for your trials.
Transparency and control –
anytime, anywhere
Key Features:
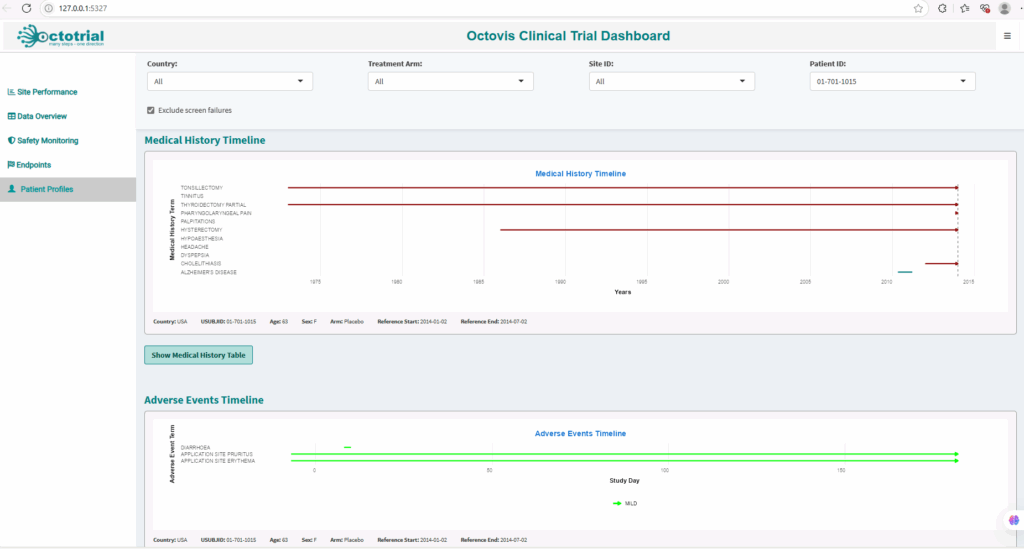
Patient Profiles:
Interactive profiles showing key data such as demographics, treatment history, and adverse events, enabling real-time tracking and safety assessments.Safety Monitoring:
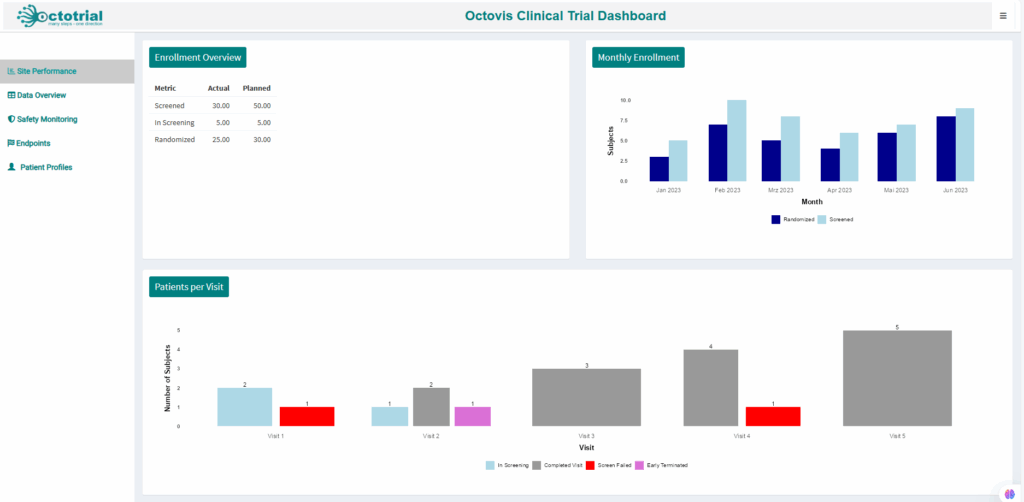
Visualize adverse events and safety trends, with early warning indicators to address potential risks quickly and ensure patient safety.Site Performance:
Track site progress with real-time metrics on recruitment, data quality, and study milestones, allowing you to optimize site performance.Audit Trail Analysis:
Access a comprehensive, audit-ready trail of all activities, ensuring full traceability and regulatory compliance according to ICH E6(R3) GCP guidelines.Study-Specific Plots & Reports:
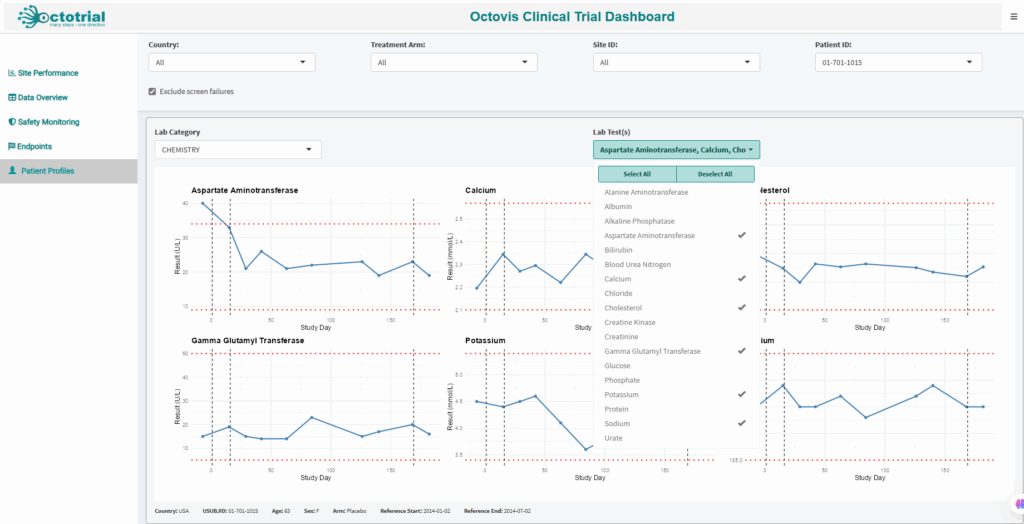
Fully customizable plots and reports tailored to your study’s specific needs, giving you in-depth insights for smarter decision-making.


Ortrud Junker-Wolf
Founder & Programmer
Data Scientist specializing in clinical research, with expertise in statistical programming, biostatistics, and data management. I help turn complex, fragmented data into actionable insights, enabling smarter, data-driven decisions. Passionate about making data work for better outcomes in clinical trials.

What to expect when
working with us
Structured thinking meets technical precision: You’ll work with someone who speaks your language – and translates it into practical solutions.
Tailored support: No one-size-fits-all system – but a solution adapted to your study design, your team, and your needs.
Reliable communication: Direct contact, clear agreements, dependable delivery.
A love of efficiency: I thrive on clean workflows, elegant visualizations, and small tools that make a big difference.
We look forward to helping you manage your trials with more intelligence, and a little more peace of mind.

Get in touch with us!
We are looking forward to hearing from you!
Please contact us: contact@octotrial.com